Per the DEA 21 CFR 1301.74b, the suspicious order monitoring requirement regulates prescription orders being filled.
Suspicious order monitoring created by the Drug Enforcement Administration requires all manufacturers, distributors and dispensers of controlled substances register with the agency. Before distributing the substance, the registrant needs to make a good faith inquiry, either with the Administration or with the appropriate State controlled substances agency to determine that person can possess the controlled substances.
Two Missions Come Together As One
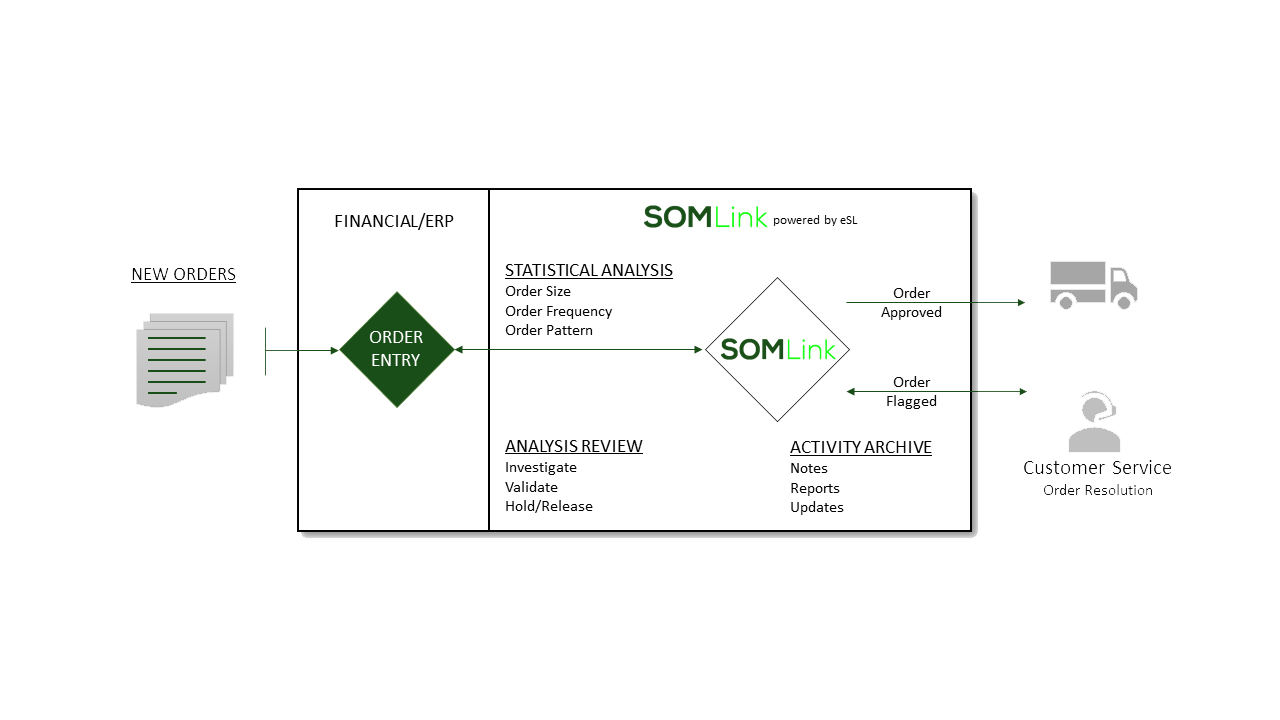
How SOMLink enforces DEA regulations:
Results: April 27, 17th National Take Back
- Total Law Enforcement Participation: 4,770
- Total Collection Sites: 6,400
- Total Weight Collected: 937,443 lbs (469Tons)
Next Take Back Day is October 26, 2019!
